Method: This prospective study was conducted in the Neonatal Intensive Care Unit (NICU), Department of Neonatology, Bangabandhu Sheikh Mujib Medical University.A total of 100 premature infants with gestational age ≤ 34 weeks were consecutively included over 9 months duration.Serum alkaline phosphatase, serum calcium and serum inorganic phosphates were measured from 1 week of chronological age until corrected term age. At corrected term age, radiologic examination was done for the assessment of osteopenia.
Results: Of the enrolled infants, 36/ 78 (46%) developed radiological evidence of osteopenia. Serum inorganic phosphate level was significantly less in osteopenic infants than non-osteopenic infants throughout first two months of life (both p value <0.001). The area under ROC curve for serum inorganic phosphate was 85% (p value 0.001). If the cut off value of serum inorganic phosphate was set at 3.6 mg/dl, then a sensitivity of 86% and a specificity of 49% were obtained.
Conclusion: Low serum inorganic phosphate at 3 weeks of life can be used as a marker for earlydetection of osteopenia of prematurity.
Key words:Osteopenia, Premature infants, Biochemical markers.
In the face of advancement in antenatal and perinatal care, every year, 15 million babies are born prematurely around the world of which more than 1 million die.1 However, the continuous developments in intensive care have led to a progressive decline in mortality.2 The achievement through an intensive care has not always ensured a favorable outcome of premature newborn.Osteopenia of prematurity (OOP) is an important co-morbiditywhich expose them to the risk of fractures and growth failure.3 Premature infants are at risk of suboptimal bone mineralization in early life, as the later part of gestational period is vital for mineral accretion.4Their bones mineral content at term corrected age has been found to be 25-70% lower than in term.5 Most of the previous studies reported that incidences of osteopenia of prematurity was as high as up to 75%.6,7 Radiologic evidence of osteopenia has been reported in 23% of very low birth weight infants and in 55% to 60% of extremely low-birth weight infants. Fractures were reported in nearly 10% of VLBW infants at 36 to 40 weeks postmenstrual age.8 Possibility of declination of incidence is anticipated over past two decades because of nutritional management.9 Recent data support its existence especially in extremely low birth weight population.3 Bangladesh is among the top 10 countries with the highest numbers of preterm birth.1 Dealing prematurity related complications have been the great challenges for the neonatal physicians. Proportion of osteopenia of prematurity was 28% according to a study done in a Bangladeshi population between the years 2003-2004.10 To date enough data are not available regarding risk factors, useful biochemical markers which can detect premature babies at risk.
Contemporary diagnosis of overt osteopenia has been based on characteristic radiological changes.11 However, the radiological changes of osteopenia are not easily detected until bone mineralization is reduced by at least 20%.12 Dual energy X-ray absorptiometry (DEXA) is considered to reflect most accurately the state of bone mineralization. Because of the rarity of DEXA instruments, a biochemical marker would be a good alternative in resource limited centers.13 Serum alkaline phosphatase, serum calcium and serum inorganic phosphates have been suggested to be a way of identifying preterm infant with osteopenia of prematurity.14 There is scarcity of evidences that any of the frequently used serum markers are valid marker of osteopenia of prematurity. Most of the authors suggested 3 weeks of life as early period of screening as alkaline phosphatase level rises in all newborns in the first 2-3 weeks of life and increases further if there is insufficient mineral supply.12 Hung Y L et al gave a conclusion that serum ALP exceeding 700IU/L at 3 weeks post natal age can predict the risk of osteopenia of prematurity12.Backstrom MC et al showed that, high serum alkaline phosphatase activity and low serum phosphate level are the best available screening method for low bone mineral density.13 A systematic review compared the results of frequently used serum and urine measurements to the results of number of imaging techniques, which showed that increased urinary calcium concentration, may be a valid biochemical marker, but more research was recommended to confirm this.11 Currently, there is neither standard recommendation for screening of osteopenia of prematurity nor data are available describing the usual values of bone markers in preterm Infants.15 Scarcity of evidences regarding screening and diagnostic tool might have lead premature infants undiagnosed subsequently subject them to the risk of growth failure. Therefore, this study was intended to examine the diagnostic performance of biochemical markers in early detection of osteopenia of prematurity.
This prospective study was conducted in the Neonatal Intensive Care Unit (NICU), Department of Neonatology, Bangabandhu Sheikh Mujib Medical University over a period of 9 months (June 2013 to February 2014). After taking informed written consent from parents, a total of 100 neonates were enrolled consequtively. Hemodynamically unstable premature infants, infants with congenital anomalies and suspected congenital bone or muscle disease were excluded.Infant's baseline demographics including gestational age (GA), birth weight (BW), small for gestational age (SGA), gender and perinatal characteristics including place of delivery, mode of delivery, Apgar score at 5 minutes were collected. Birth weight was recorded from labor room or neonatal referral sheet in case of out born infants. Admission weight was taken by a digital weighing scale (SALTER, Model-914). Gestational age was assessed by LMP, sonographic findings and modified new ballard scoring. Postnatal medical records (respiratory distress syndrome, sepsis, and retinopathy of prematurity, time to full feeding) were recorded.
Blood samples for biochemical tests were done biweekly from 1 week postnatal age onwards up to corrected term age. During study period, these biochemical markers were incorporated into routine biochemical follow-up of premature infants and sample were taken aseptically adjusting with other purposes of venepuncture. Frequency was at least 3 in infants with gestational age ≥33 weeks, 4 in ≥ 31 weeks, 5 times in ≥ 29 weeks. Serum alkaline phosphatase activity was measured by colorimetrically by p-nitrophenyle phosphorus acid radical quality method. It was reported in U/L and normal reference value for adult was 50-136 U/L. A prefeed blood sample in a plain tube was used. Serum calcium was examined by the complexed cresolephthalein test. A Level of < 7 mg/dl was taken as low calcium value for the newborn infant. Serum inorganic phosphate was measured using a direct phosphomolibdate reaction, which can be measured colorimetrically. Normal reference value was taken as 3.0-4.5 mg/dl.Enrolled premature infants underwent for radiologic examination of forearm with portable X-ray machine at corrected term age. Radiologic examination of forearm with portable X ray machine was performed by single radiographer. Infants were exposed to radiation dose at 50-55 kVp (kilovolt peak) and 0.5 mAs (milliamp seconds). Diagnostic reference level was 68 kVp / 0.5mAs. Comments on radiographic findings were made by a radiologist who was blinded to laboratory findings. The scoring method of Koo et al17 was used to assess osteopenia. Grade 0 has been defined as findings of normal bone, grade 1 as mineral rarefaction, grade 2 as fraying and cupping of the metaphyses and grade 3 as fractures. Osteopenia is considered when there is radiographic evidence of diminished bone density defined as ≥ grade 1. Patient's outcomes including presence of osteopenia, length of hospital stay, gestational age at radiologic examination, gestation were documented.
Study was approved by Institutional Review Board (IRB) of Bangabandhu Sheikh Mujib Medical University. Data were compared between osteopenic and non-osteopenic using Chi Squared test for categorical variables and Independent-t test for continuous variables. Receiver operator characteristics (ROC) curves were used to determine the individual diagnostic performance of biochemical bone markers. The statistical software IBM SPSS Statistics version 19 (SPSS Chicago, IL) was used for the statistical analysis. Results were considered statistically significant at ,i>p value < 0.05.
During the study period, 190 eligible preterm infants ≤ 34 weeks gestation were admitted in the Neonatal Intensive Care Unit. After taking informed written consent, a total of 100 neonates were enrolled. Three parents denied giving blood for serum bone biochemical markers estimation and withdrew consent, 9 patients died during study period (6 during hospital stay, 3 after discharge from hospital) and 10 patients did not complete the follow up and radiological diagnosis. The remaining 78 infants were ultimately retained for analysis.Demographic and perinatal characteristics of studied infants are presented in table I. Of the 78 infants, 36 (46%) developed radiological evidence of osteopenia at corrected term age. Of them 22 (28%) infants were grade 1, 13 (17%) were grade 2, remaining 1 (1%) was grade 3 osteopenia according to Koo's criteria.17 Mean gestational age of radiologic detection of osteopenia of prematurity was 39 ± 1.3 weeks. According to the gestational age category, osteopenia was significantly more frequent (22/31, 71%) in less than 32 weeker premature infants in comparison to infants with gestational age ≥32 weeks. Comparison of demographic and clinical characteristics in premature infants who had osteopenia and those without osteopenia at corrected term age is shown in table II. Compared with non-osteopenic infants, infants with osteopenia were significantly smaller (p value 0.001) and more premature (p value <0.001). Infants with osteopenia suffered from sepsis significantly more frequently than non-osteopenic infants (p value 0.01). Premature infants with osteopenia tended to have significant delay in achieving full feed (p value 0.001) than that of infants without osteopenia. Significant difference was also found in case of need for mechanical ventilation (p value 0.028). Hospital stay was also significantly prolonged in osteopenic preterm infant than that of non-osteopenic infants (p value 0.001).
Variable Infants (n=78) |
Birth weight (g) 1384±223 |
Numerical data are presented as mean ± sd and categorical data as percentage (%)
Variable |
Osteopenic(n=36 ) |
Non-osteopenic(n=42) |
pValue |
Gestational age(wks) |
31.17 ± 1.50 |
32.60 ± 1.49 |
49 <0.001 |
Birth weight(g) |
1295 ± 189 |
1460 ± 224 |
0.001 |
Male(%) |
18 (50%) |
23 (55%) |
0.30 |
Small for gestational age |
9 (25%) |
6 (14%) |
0.04 |
Sepsis |
16 (44%) |
8 (19%) |
0.01 |
Time to full feed(d) |
20 ± 8.17 |
4 ± 6.7 |
0.001 |
Need for MV |
6 (16%) |
|
1 (3%) |
|
0.02 |
|
|
RDS |
8 (22%) |
|
6 (14%) |
|
0.36 |
|
|
ROP |
6 (16%) |
5 (12%) |
0.42 |
|
0.42 |
|
|
Length of hospital stay(d) |
27 ± 10.7 |
18 ± 9.0 |
<0.001 |
Comparison of various biochemical markers of osteopenia of prematurity among osteopenic and non-osteopenic infants are demonstrated in table III.
Variable |
Osteopenic |
Non-osteopenic(n =42) |
p Value |
Serum Alkaline Phosphatase |
|||
1-4 weeks |
224 ± 48 |
210 ± 42 |
0.23 |
5-8 weeks |
494 ± 138 |
274 ± 60 |
< 0.001 |
Serum inorganic Phosphate |
|||
1-4 weeks |
3.9 ± 0.49 |
4.8 ± 0.69 |
<0.001 |
5-8 weeks |
3.2 ± 0.80 |
4.6 ± 0.50 |
<0.001 |
Serum calcium |
|||
1-4 weeks |
8.2 ± 0.31 |
8.4 ± 0.51 |
0.06 |
5-8 weeks |
8.1 ± 0.37 |
8.2 ± 0.79 |
0.7 |
Biochemical data were available for 255/295 (87%) of the study population. Of 255 sets of data, biochemical data of 78 infants were available in 1 week of age, 63 data were collected at 3 weeks of age, 60 data were available at 5 weeks of age and 54 data were documented at 7 weeks of age. As biochemical tests could not be done strictly on biweekly basis, biochemical data were summarized in two time period; 1-4 weeks and 5-8 weeks. The proportion of missing biochemical data were similar between the two groups (26/150 [17.3%] vs 18/100 [18%], p value 0.39). Serum alkaline phosphatase level was significantly elevated in 5-8 weeks of life (p value <0.001) in osteopenic infants when compared with non-osteopenic infants. No significant difference in serum alkaline phosphatase level could be demonstrated between two groups in 1-4 weeks of life. Serum inorganic phosphate level was significantly less in infants with osteopenia than without osteopenia both in1-4 weeks (p value <0.001) as well as 5-8 weeks of life (p value <0.001). No differences were found between osteopenic and non-osteopenic infants regarding serum calcium level in both time periods.
Receiver Operator Characteristic (ROC) curves was constructed to determine individual diagnostic performance at 1 and 3 weeks post-natal age in predicting the occurrence of osteopenia at corrected term age (figure 1).
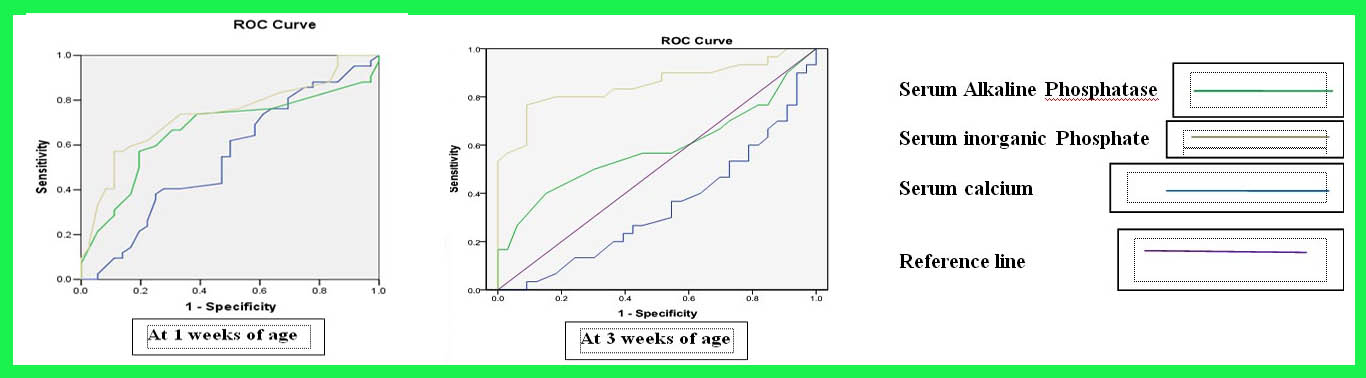
Figure 1: Receiver operator characteristic curves of biochemical markers at 1 and 3 weeks postnatal age in detecting the risk of osteopenia at term age.
Predictive accuracy of serum inorganic phosphate was good and statistically significant in comparison to serum alkaline phosphatase and serum calcium in both the curves. Predictive accuracy of inorganic phosphate was more in 3 weeks curve as the area under ROC curve for serum inorganic phosphate was 85% (p value 0.001) at 3 weeks of age. At 3 weeks age the areas under ROC curve for serum calcium and serum alkaline phosphatase were 58% and 34% respectively (p value 0.29 and 0.06 respectively). If the cut off value of serum inorganic phosphate was set at 3.6 mg/dl, then a sensitivity of 86% and a specificity of 49% were obtained.
Discussion
Serum alkaline phosphatase, serum inorganic phosphate and serum calcium have been used to screen, early detection and prediction of osteopenia in the premature infants. In the first 2-3 weeks of life, with the physiological remodeling, alkaline phosphatase level raises in all newborns and increase further if there is insufficient mineral supply. Newborns receiving sufficient nutrition may have only smaller rises in alkaline phosphatase.18 This is the explanation behind the reason of taking biochemical sample at 3 weeks of age by most of the author. In this study, biochemical markers at 3 weeks of age were used to determine predictability of osteopenia at corrected term age.Receiver operator characteristic (ROC) curves were constructedto examine diagnostic performance of bone marker at 3 weeks of age in predicting the osteopenia at corrected term age. The area under the ROC curve for serum inorganic phosphate was high and statistically significant in comparison to serum alkaline phosphatase and serum calcium. Best cut-off value was obtained at 3.6 mg/dl yielded sensitivity 86% and specificity 49%. Backstrom et al13 attempted to enhance detection rate of osteopenia by combining serum alkaline phosphatase and serum phosphate. They found that serum phosphate level below 1.8 mmol/l demonstrate 50% sensitivity and 95% specificity at 3 months corrected age. They further combine serum alkaline phosphatase along with serum phosphate and reach 100% sensitivity and 70% specificity at 900 U/l and 1.8 mmol/l values respectively.Yi-Li Hung et al12 found striking dissimilarity that in spite of significant difference in the level of the serum phosphorus between osteopenic and non-osteopenic infants at 3 weeks of age, they could not demonstrate predictive accuracy of serum phosphate. While comparing diagnostic performance of serum alkaline phosphate, serum inorganic phosphate, serum calcium at 3 weeks of age, predictive accuracy of serum inorganic phosphate was considerably smaller than those of serum alkaline phosphatase according to their study findings. They concluded that serum ALP concentration exceeding 700 IU/L at 3 weeks post-natal age can predict the risk of osteopenia in preterm infants.
The result of this study showed, serum alkaline phosphatase level was significantly elevated only in 5-8 weeks of life in case of osteopenic infants when compared with non osteopenic. There was no significant difference in serum alkaline level between two groups in 1-4 weeks of life, suggesting that serum ALP level is not a good marker of osteopenia in first 4 weeks of life. Serum inorganic phosphate level was significantly less in osteopenic infants than non-osteopenic group in first two months of life, indicating that a persistently low phosphate level contributes to the development of osteopenia of prematurity. A fall in plasma phosphate concentration has been described in very low birth weight infants who developed disturbances in the mineralization, especially among those receiving human milk.13,16 This might be the explanation of the finding regarding changes in the serum phosphate in the present study. A retrospective study of 230 conducted by Sreekanth et3 demonstrated similar findings with that of present study with the exception that they have investigated biochemical markers among extremely low birth weight population. To date role of biochemical markers in detection of osteopenia of prematurity are questionable. Utility of serum alkaline phosphatase and serum phosphate in the detection of osteopenia of prematurity were challenged by J Faerk et al14 as they could not demonstrate any association between bone mineral content and mean alkaline phosphatase and serum phosphate. The role of urinary calcium and phosphorus levels were investigated along with serum calcium and phosphorus levels by catache and Leone.19 They argued that serum calcium and phosphorus levels are not good markers in early detection of mineral deficiency, rather urinary calcium level may be helpful in early detection of mineral deficiency.
In this study of a recent cohort of premature babie's, 46% (36/78) had radiological evidence of osteopenia at corrected term age. The incidence is similar to that has been previously reported in literature. In 1989, Koo et at17 observed 30% incidence of fractures/rickets in first 1 year of life while following 78 preterm infants. Mitchell et al15 selectively screened 32 extremely low birth weight infants with ALP level > 800 IU/L for radiological rickets. All had evidence of osteopenia and 18/32 (56%) had radiological rickets. In a recent study by Yi-Li Hung et al12 , concluded that osteopenia of prematurity among ≤ 34 weeker was 39%. A retrospective review by Burm seok et al20 between 2009 and 2011 revealed 43% (112/258) incidence of osteopenia.
During the study period radiology was the only tool to make definitive evidence of osteopenia. So, radiation hazard of X-ray could not be avoided in studied infants. In standard radiograph, the bone changes of osteopenia of prematurity are a late sign as these appear only after 20-30% of bone mineral content is lost. So, it was possible to miss the premature infants with mild osteopenia.
Conclusion: Radiologic evidence of Osteopenia was 46% among premature infants gestational age ≤ 34 weeks and inversely proportional to the degree of gestational age. Low serum inorganic phosphate at around 3 weeks of life can be used as a predictor of osteopenia of prematurity. Future research is needed to find out a reliable investigation tool to make correct and early diagnosis.
Acknowledgment
We gratefully acknowledge the sincere cooperation of the parents as well as the contribution of all doctors, staffs of the department of neonatology, obstetrics and gynaecology and the department of radiology of BSMMU
References
- Blencowe H, Cousens S, Oestergaard M et al. National, regional and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. The Lancet, Jun 2012; 379(9832):2162-72.
- Linda L Wright, Betty R Vohr, Avroy A Fanaroff. Perinatal-Neonatal Epidemiology. Perinatal, mortality and morbidity information for infants born in the National Institute of child Health and Human Development (NICHD) Neonatal Research Network from 1997 to 2000. Avery's disease ofthe Newborn8th edition. Elsevier Saunders; 2004: 3-5.
- Viswanathan S, Khasawneh W, McNelis K, et al. Metabolic Bone Disease: A Continued Challenge in Extremely Low Birth Weight Infants. J Parenter Enteral Nutr 2014 Nov; 38(8):982-90.
- Fewtrell M.Early nutritional predictors of long-term bone health inpreterm infants. Curr Opin in Clin Nutr and Metab Care 2011; 14(3): 297-301.
- Horsman A, Ryan SW, Congdon PJ, Truscott J G and James J R. Osteopenia in extremely low birth weight infants. Archives of Disease in Childhood 1989; 64: 485-8.
- Crofton PM, Shrivastava A, Wade JC, et al. Bone and collagen markers in preterm infants: relationship with growth and bone mineral content over the first 10 weeks of life. Pediatric Research 1999; 46: 581-7.
- Lyon AJ, McIntosh N, Wheeler K, Williams JE. Radiological rickets in extremely low birth weight infants. Pediatr Radiol 1987; 17(1): 56-8.
- Vachharajani AJ, Mathur AM, Rao R. Metabolic Bone Disease of Prematurity. Neo Reviews2009;10: e 402-e411.
- Bozzetti V, Tagliabue P. Metabolic bone disease in preterm newborn: an update on nutritional issue. Ital J Pediatr. 2009; 35(1): 20.
- Afroze N. Osteopenia in premature infants and effect of calcium and phosphorus supplementation on it. FCPS dissertation, Bangladesh College of Physician and Surgeon, Dhaka, January 2007.
- Visser F, Sprij A J, Brus B. The validity of biochemical markers in metabolic bone disease in preterm infants a systematic review. Acta Paediatrica 2012; 101(6): 562-568.
- Hung YL, Chen PC, Jeng SF, et al. Serial measurements of serum alkaline phosphatase for early prediction of osteopaenia in preterm infants. J Paediatr Child Health. 2011; 47(3): 134-9.
- Backstrom MC, Kouri T, Kuusela AL, et al. Bone isoenzyme of serum alkaline phosphatase and serum inorganic phosphate in metabolic bone disease of prematurity. Acta Paediatrica 2000; 89(7): 867-873.
- Faerk J, Peitersen B, Petersen S, Michaelsen K F. Bone mineralisation in premature infants cannot be predicted from serum alkaline phosphatase or serum phosphate. Arch Dis Child Fetal Neonatal Ed 2002; 87: 133-136.
- Mitchell SM, Rogers SP, Hicks PD, Hawthorne KM, Parker BR and Abrams SA. High frequencies of elevated alkaline phosphatase activity and rickets exist in extremely low birth weight infants despite current nutritional support.BMC Pediatrics2009; 9: 47.
- Backstrom MC, Kuusela AL, Maki R. Metabolic bone disease of prematurity.Ann Med 1996; 28(4): 275-82.
- Koo WW, Gupta JM, Nayanar VV, Wilkinson M and Posen S. Skeletal changes in preterm infants. Archives of Disease in Childhood1982; 57: 447-52.
- Tinniton JR, Embleton DN. How to use... alkaline phosphatase in neonatology. Arch Dis Child Educ Pract Ed 2012; 97: 157-163.
- Catache M, Leone CR. Role of plasma and urinary calcium and phosphorus measurements in early detection of phosphorus deficiency in very low birth weight infants. Acta Paediatrica. 2003; 92(1): 76-80.
- Oh BS, Choi JS, Kim YN, Song ES, Choi YY. Usefulness of serum alkaline phosphatase in predicting osteopenia and rickets in very low birth weight infants. J Korean Soc Neonatal 2012; 19(4): 229-236.
- Soc Neonatal 2012; 19(4): 229-236