1Department of Haematology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh; 2Department of Paediatric Haematology & Oncology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
 10.3329/bmrcb.v45i2.42546
10.3329/bmrcb.v45i2.42546  0000-0001-8903-6672
0000-0001-8903-6672
Autoimmune haemolytic anaemia (AIHA) is an uncommon disorder characterized by shortened red blood cell (RBC) survival and the presence of autoantibodies directed against autologous RBCs.1 It presents with anaemia, jaundice, and splenomegaly with reticulocytosis, unconjugated hyperbilirubinaemia, and direct antiglobulin test (DAT) positivity.2 AIHA has an estimated incidence of 1–3 cases per 100,000 subjects per year, a prevalence of 17:100,000 and a mortality rate of 11%.3-5 AIHA is classified as warm AIHA (75.0% of all AIHA cases), cold AIHA (about 15.0%), and mixed type AIHA (less than 5.0%), based on the thermal range of autoantibodies involved in the pathogenesis.6 It can be idiopathic (50.0%) or secondary to lymphoproliferative syndromes (20.0%), autoimmune diseases (20.0%), infections, tumors and drugs.7 The laboratory diagnosis of AIHA depends on the result of direct antiglobulin test (DAT) which shows positivity with anti-IgG (usually in warm AIHA) and/or anti-C3d (usually in cold AIHA) antisera, and also the presence of laboratory findings supporting hemolysis such as increase of serum lactate dehydrogenase (LDH), reticulocytosis and spherocytosis in peripheral blood smears.8 The treatment of AIHA is still not evidence-based. The first-line therapy for warm AIHA is corticosteroids, which are effective in 70.0-85.0% of patients and should be slowly tapered over a time period of 6-12 months. For refractory/relapsed cases, the current sequence of second-line therapy is splenectomy (effective approx. in 2 out of 3 cases but with a presumed cure rate of up to 20.0%), rituximab (effective in approx. 80.0-90.0% of cases), and thereafter any of the immunosuppressive drugs (azathioprine, cyclophosphamide, cyclosporin, mycophenolate mofetil). Additional therapies are intravenous immunoglobulins, danazol, plasma-exchange, and alemtuzumab and high-dose cyclophosphamide as last resort option.4 Most of the immunosuppressive drugs have many deleterious side-effects and the onset of action is slow. Splenectomy carries the risk of an overwhelming post splenectomy sepsis.8 Rituximab (so called “medical splenectomy”) is an effective second-line therapy, can work quickly with lasting effects, providing high initial response rates up to 87.0% and prolonged disease free survivals of 56.0% up to 2 years with reduced adverse reactions.8-10 For warm AIHA, rituximab therapy is considered as a second line option as monotherapy or combined therapy. It can also be used as a first line therapy in combination with steroids for newly diagnosed patients.11 Another option, with less supportive evidence in the literature, is the use of therapeutic plasma exchange (TPE) to quickly reduce the pathologic antibody titer in patients with an overwhelming hemolysis.12 TPE has been performed in a relatively small number of severely affected warm AIHA patients in whom the anemia could not be stabilized with steroids and transfusion therapy alone, as a temporizing measure which seemed to stabilize the disease and increase the efficiency of blood transfusions.4 Current indication categories endorsed by the American Association of Blood Banks (AABB) and the American Society for Apheresis, TPE for AIHA is considered as acategory III indication, i.e. an application representing “heroic or last-ditch efforts on behalf of a patient’’.13 Decision to transfuse RBC in AIHA should be based on the clinical condition of the patient. No critical patient should be denied blood transfusion due to serological incompatibility and “the least incompatible” RBC should be transfused in this situation.4,8,14,15 Severe AIHA cases sometimes misdiagnosed or lately diagnosed. Early diagnosis and appropriate emergency interventions can save the life of these patients. In this case, we demonstrate the concomitant use of TPE and rituximab in the treatment of life-threatening warm AIHA.
Keywords: Autoimmune haemolytic anaemia, Corticosteroid, Therapeutic plasma exchange, Rituximab
Aminul Haque, 67-year-old man, known case of DM, admitted in Lab one hospital, Dhaka on the 3rd December 2017, with 7 days history of weakness, bodyache, fever, dizziness, fatigue and progressive shortness of breath. He denied any history of trauma, evidence of GIT bleeding, drug ingestion, previous history of anaemia, haemoglobinopathy or kidney disease. On physical examination, the patient was febrile, tachycardic, severely anaemic, mildly icteric and just palpable splenomegaly. Laboratory findings showed: Haemoglobin 5.7 g/dl; ESR 140 mm in 1st hour ; WBC: TC 18.5×109/L, Neutrophil 80%, Lymphocyte 15%; Platelet 295×109/L ; PBF : RBC showed spherocytosis, clumping & frequent nRBC; Total S. bilirubin (total) 5.8 mg/dl; S. LDH markedly raised (2749 U/L); ALT 46 U/L; AST 92U/L; S. Alkaline phosphatase 299 U/L; Renal function was normal; RBS: 18mmol/l; USG: mild splenomegaly; DAT: strongly positive; IAT: weakly positive; S. protein electrophoresis: polyclonal gammopathy; Bone marrow study: Dimorphic erythroid hyperplasia.
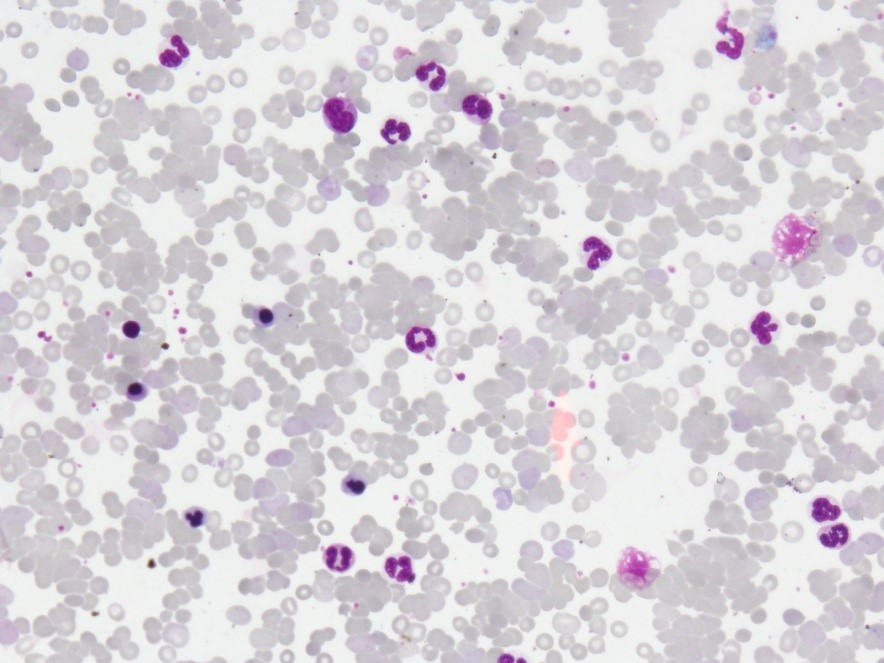
Figure 1: PBF showing spherocytosis, clumping, frequent nRBC & neutrophilic leukocytosis
With these findings, the patient was diagnosed as a case of warm AIHA. Treatment was started with high dose corticosteroid (Dexamethasone 5mg i/v 8hourly). Out of 10 donors, only 1 was found least incompatible and thus only 1 unit of RCC can be transfused to the patient. A further decrease in hemoglobin was observed on day 3 of corticosteroid therapy and blood glucose level was rising despite of insulin therapy. Further RCC transfusion was not possible because of donor incompatibility. The patient’s clinical condition was gradually becoming worsened significantly. TPE was therefore started under intensive care monitoring on day 4. TPE was performed on every alternate day for 5 times. The patient was in better clinical condition after the procedures. There after 2 units of RCC was transfused. After that he was haemodynamically stable and has not shown any further hemolytic phenomena. Then azathioprine (50mg b.d) was added and tapering of dexamethasone had to be started because of uncontrolled DM. But few days later, haemoglobin was again gradually decreasing and ESR was rising. Then Inj. Rituximab (375mg/m2 weekly for 4 weeks) was started along with azathioprine. After the 1st dose of rituximab, he was gradually improving and successfully completed remaining 3 doses, without any major adverse reactions with further improvement of clinical conditions. Since then, no further transfusion support was required and the patient was then on azathioprine only.
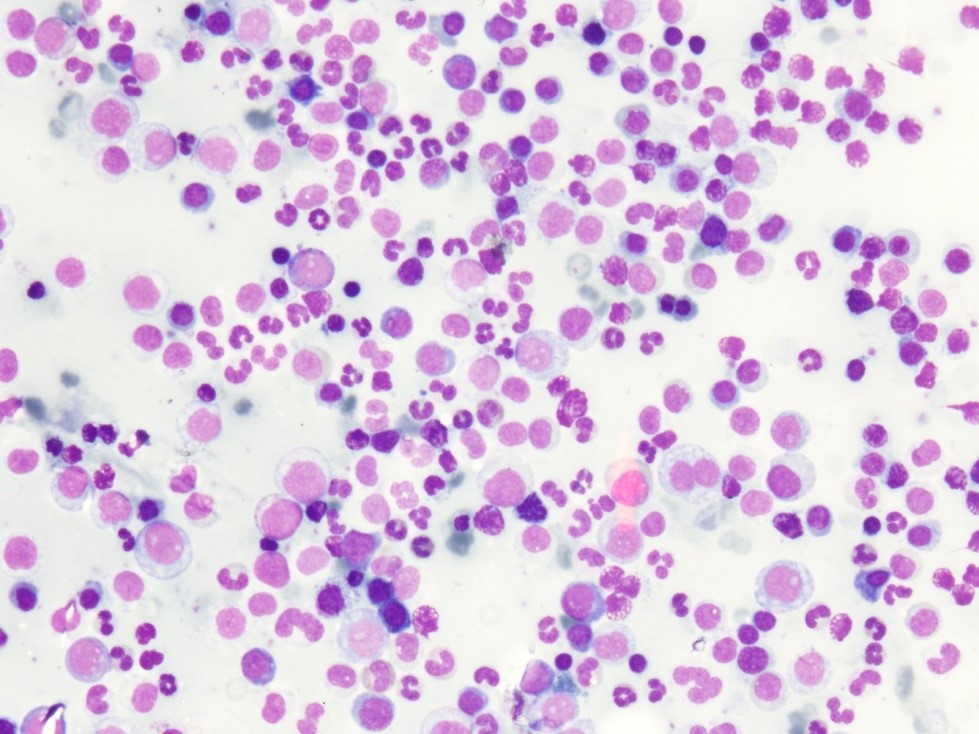
Figure 2: Bone marrow showing Dimorphic erythroid hyperplasia
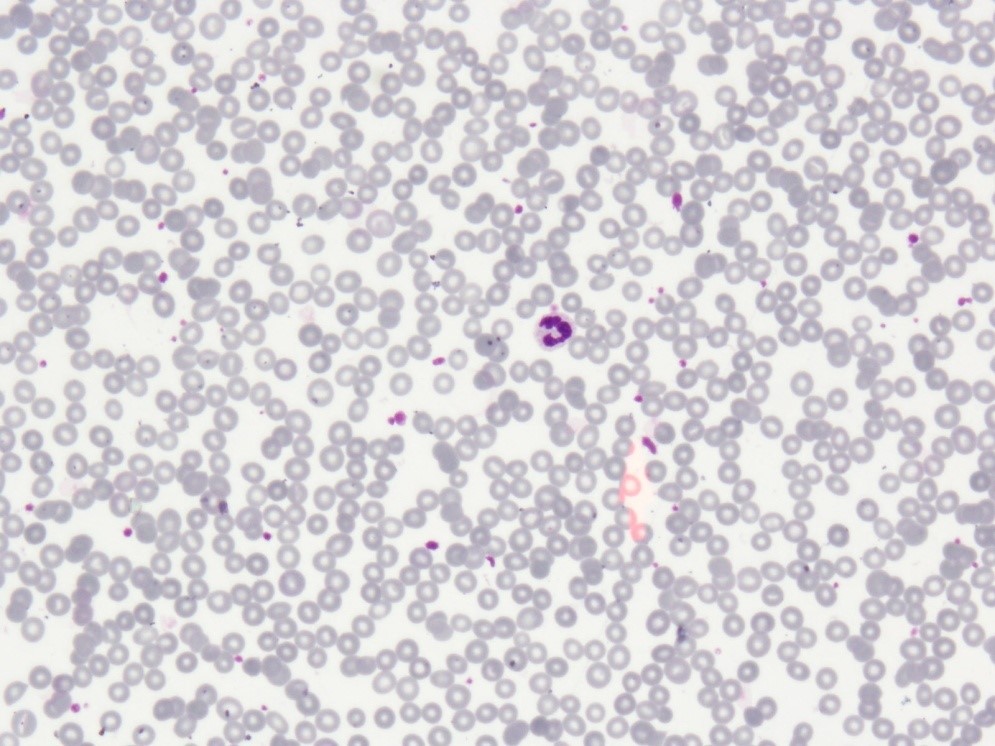
Figure 3: Normal PBF findings after completion of treatment
Plasma exchange has been used in the therapy of a wide variety of disorders, particularly those associated with a well-documented or suspected immunologic pathogenesis. It sounded rationale to use plasma exchange to remove antibodies as a supportive treatment to sustain the vital functions of the patient, until immunosuppressive therapy became effective. The American Society for Apheresis suggests the usage of TPE in AIHA as an emergency measure. TPE can be used to maintain acceptable haemoglobin levels, remove free haemoglobin, reduce the titre of auto-antibodies and sustain cardiopulmonary functions.13 Rituximab therapy is considered as a second line option as monotherapy or combined therapy. It can also be used as a first line therapy in combination with steroids for newly diagnosed patients. Therapy with rituximab is well tolerated, effective and has a shorter time of onset compared to the other immunosuppressive agents and provides a prolonged remission with a relatively short course of steroid therapy.
The patient had severe disease, manifested by the rapid development of anemia in less than 96h, minimal reticulocytosis, haemoglobinuria and a poor response to conventional therapy. It was TPE as an emergency measure which controlled the overwhelming hemolysis. Then rituximab therapy provided the sustained response.
Conclusion
In summary, the patient presented with a severe, life-threatening AIHA not responsive to conventional therapy. TPE was initiated on emergency basis to quickly and temporarily reduce the over whelming titre of pathologic antibody. This was demonstrated by an improved reticulocytosis and a reduction in the net change in hemoglobin following TPE. Rituximab was added as an immunosuppressant that provided prolonged remission from the overwhelming haemolysis. The combination of all the modalities resulted in the successful management of this severe case of AIHA.
References
- Packman CH. Hemolytic anemia resulting from immune injury. In: Kaushansky K, Lichtman MA, Prchal JT, Levi M, Press OW, Burns LJ, Caligiuri MA, editors. Williams Hematology. 9th ed. New York: McGraw-Hill Education; 2016. p. 823.
- De Gruchy GC. The haemolytic anaemias. In: Firkin FC, Chesterman CN, Penington DG, Rush BM, editors. De Gruchy’s Clinical Haematology in Medical Practice. 5th ed. Edinburgh: Blackwell Science Ltd.; 1989. p. 172‑215.
- Eaton WW, Rose NR, Kalaydjian A, Pedersen MG, Mortensen PB. Epidemiology of autoimmune diseases in Denmark. Journal of autoimmunity. 2007 Aug 1;29(1):1-9.
- Zanella A, Barcellini W. Treatment of autoimmune hemolytic anemias. Haematologica. 2014 Oct 1;99(10):1547-54.
- Go RS, Winters JL, Kay NE. How I treat autoimmune hemolytic anemia. Blood. 2017 Jun 1;129(22):2971-9.
- Bass GF, Tuscano ET, Tuscano JM. Diagnosis and classification of autoimmune hemolytic anemia. Autoimmunity reviews. 2014 Apr 1;13(4-5):560-4.
- Valent P, Lechner K. Diagnosis and treatment of autoimmune haemolytic anaemias in adults: a clinical review. Wiener klinische Wochenschrift. 2008 Mar 1;120(5-6):136-51.
- Park SH. Diagnosis and treatment of autoimmune hemolytic anemia: classic approach and recent advances. Blood research. 2016 Jun 1;51(2):69-71.
- Zecca M, De Stefano P, Nobili B, Locatelli F. Anti-CD20 monoclonal antibody for the treatment of severe, immune-mediated, pure red cell aplasia and hemolytic anemia. Blood. 2001 Jun 15;97(12):3995-7.
- Webster D, Ritchie B, Mant MJ. Prompt response to rituximab of severe hemolytic anemia with both cold and warm autoantibodies. American journal of hematology. 2004 Apr 1;75(4):258-9.
- Rodrigo C, Rajapakse S, Gooneratne L. Rituximab in the treatment of autoimmune haemolyticanaemia. British journal of clinical pharmacology. 2015 May 1;79(5):709-19.
- Aglieco F, Manickaratnam S, Bona R, Kaplan AA. A case report of refractory warm autoimmune hemolytic anemia treated with plasmapheresis and rituximab. Therapeutic Apheresis and Dialysis. 2008 Apr 1;12(2):185-9.
- Smith JW, Weinstein R, Hillyer KL, AABB Hemapheresis Committee. Therapeutic apheresis: a summary of current indication categories endorsed by the AABB and the American Society for Apheresis. Transfusion. 2003 Jun 1;43(6):820-2.
- Park SH, Choe WH, Kwon SW. Red blood cell transfusion in patients with autoantibodies: Is it effective and safe without increasing hemolysis risk?. Annals of laboratory medicine. 2015 Jul 1;35(4):436-44.
- Das SS, Zaman RU, Safi M. Incompatible blood transfusion: Challenging yet lifesaving in the management of acute severe autoimmune hemolytic anemia. Asian journal of transfusion science. 2014 Jul;8(2):105