1Department of Pediatrics, Anwer Khan Modern Medical College Hospital, Dhaka, Bangladesh; 2Department of Neonatal Medicine & NICU, BICH & Dhaka Shishu (Children) Hospital, Dhaka, Bangladesh; 3Department of Neonatology, NICU &Critical care Pediatrics, BICH & Dhaka Shishu (Children) Hospital, Dhaka, Bangladesh; 4Department of Neonatal Cardiology, Department Pediatric Cardiology, BICH & Dhaka Shishu (Children) Hospital, Dhaka, Bangladesh
 10.3329/bmrcb.v45i3.44651
10.3329/bmrcb.v45i3.44651  0000-0002-9188-4942
0000-0002-9188-4942
Background: Pulse oximetry is a noninvasive technique of measuring oxygenation of the blood that is used worldwide to assess critically ill patients. The accuracy of pulse oximetry reading may be related to the site of pulse oximeter probe placement; but this may be difficult in sick neonates. In neonates, palm and sole are commonly used site for probe placement.
Objective: This study was conducted to assess the accuracy of pulse oximeter oxygen saturation (SpO2) from probe placement at wrist and ankle as an alternative to palm and sole in neonates.
Methods: This cross sectional study was carried out in SCABU of Dhaka Shishu (Children) Hospital from December 2012 to March 2013. A total of 169 neonates were enrolled in this study. Two pulse oximeters were used for simultaneous paired SpO2 measurements. The SpO2 was measured at 0 sec, then at 30 sec and at 1 min over the palm and ipsilateral wrist, both side and repeated the same procedure over the sole and ipsilateral ankle, both side and were recorded in the case record form. Two tailed, Student’s t-test was performed for analysis of continuous, normally distributed variables. Regression analysis was performed to determine the relationship between paired SpO2 measurements.
Results: A total of 169 patients (birth weight 2530.8±772.2 g, gestational age 36.7±3.9 weeks, mean age 7.7 days and age range 1-27 days) were enrolled. There was a good correlation between SpO2 measured at the palm versus the wrist (r= 0.92, p<0.0001 (right); r= 0.88, p< 0.0001 (left)) and between SpO2 measured at the sole versus the ankle (r=0.90, p<0.0001 (right); r= 0.98, p<0.0001 (left)). There was also a good agreement between paired SpO2 measurements from these sites. The bias and precision for SpO2 at the right palm and right wrist was 0.08 ± 0.65% and for the left palm and left wrist 0.05 ± 0.79%. Similarly, the bias and precision for SpO2 at the right sole and right ankle was -0.11 ± 0.63% and for the left sole and left ankle was 0.56 ± 0.32%.
Conclusions: The wrist and ankle can be used as alternative sites, to measure SpO2 in newborn infants in place of the routinely used palm or sole.
Keywords: SpO2; Pulseoximetry; Newborn; WristPulse oximeter arterial oxygen saturation (SpO2) has become the "fifth vital sign" in the examination of every newborn and infant with respiratory distress, besides temperature, pulse, respiration and blood pressure.1-3 Pulse oximetry is noninvasive, easy to use, has no side effects, is accurate and allows continuous monitoring and is the preferred method of oxygen monitoring in neonate.4,5 Pulse oximeter estimates arterial oxygen saturation by measuring the absorption of light in human tissue beds and light is absorbed in various degrees by tissue, bone, blood vessels, fluids, skin, venous blood, arterial blood, including various types of hemoglobin. The background absorption changes when there is changes in the shape or position of the tissue through which the light passes6. So, the choice of probe site may affect accuracy of pulse oximeter reading.7
The ideal site for placement of probe is one that is well perfused, relatively immobile, comfortable for the patients and easily accessible. The ear lobes and fingers are the commonly used sites.8 In infants flexible probe work through palm, sole, penis, wing of nostril, wrist and ankle.9,10The choice of probe site may be difficult in sick neonates, who have intravenous lines for fluid infusion, or heparin locks for medication infusions on the dorsum of the hands and the feet. Wrist or ankles have been an alternative site for pulse oximeter probe location in these critically ill neonates requiring oxygen saturation monitoring.10 Moreover, the performance of the pulse oximeter can be disrupted by several factors including poor peripheral perfusion, peripheral vasoconstriction, hypotension, low pulse pressure and patients motion.11 On the other hand, prolong placement of probe can cause mechanical injury (e.g., finger stiffness), ischaemic pressure necrosis, burning or blistering of the placement site.8 So, alternative site for probe placement is needed in critically ill and sick neonates. Little is known about the accuracy of pulse oximetry when the probe is placed at the wrist and ankle. This study was undertaken to see whether the wrist and ankle can be the alternative site for pulse oximeter probe placement in sick newborn to monitor oxygen saturation.
This cross sectional study was carried out in SCABU of Dhaka Shishu (Children) Hospital from December 2012 to March 2013. The study was approved by Ethical Review Committee, Bangladesh Institute of Child Health, Informed written consent was obtained from the parents.
A total of 169 neonates were enrolled in this study. Purposive, and convenient sampling was done. Sample size has been calculated using the formula:
\[n = {Z^2pqN\over e^2(N-1)+Z^2pq} \]Here, Z = 1.96 [at 5% level of significance or 95% confidence interval (CI)]
p = 50% (0.5) [as prevalence is not known]
q = (1 - p) = 50% (0.5), e = 10% of p (0.5)= 0.05
N = 300 (as hospital record showed that total SCABU admission of neonate is on an average 300 in 3 months)
So, n=168.6 = 169
Newborns with 0 to 28 days, both male, female and both term and preterms were included in this study. Neonates in shock and those with core body temperature <350C, getting ionotrop at high dose (Dopamin/Dobutamine ³10µg/kg/min) and neonates with multiple congenital malformations were excluded from the study.The baseline data including age, sex, birth weight, current weight, temperature, respiratory rate, heart rate, signs of respiratory distress, requirement of oxygen and medication were recorded in the questionnaire.
The finger print pulse oximeter, manufactured by Smiths Medical PM, Inc, USA was used for this study. Recording of SpO2 was carried out by two oximeters simultaneously. The SpO2 measurements at the palm and ipsilateral wrist were recorded at 0 sec, then at 30sec, and at 1 min, on both side and the same procedure was done to record SpO2 measurements over the sole and ipsilateral ankle of both sides. During measurement of SpO2 one assistant auscultates the heart rate and the value of SpO2 should be considered only if the pulse oximeter pulse rate not differs by more than three beats from the heart rate. In neonates receiving phototherapy, the lights were temporarily turned off during the procedure.
Data analysis was done by using SPSS version 17, two-tailed Student’s t-tests done for analysis of continuous, normally distributed variables. Regression analysis was performed to determine the relationship between paired SpO2 measurements. In addition, the mean and standard deviation of the paired SpO2 differences (bias and precision) was calculated by using Bland-Altman analysis in MedCalc Software 2014. p values less than 0.05 was consider as statistically significant for all tests.
Among the enrolled 169 neonates, mean age at admission was 7.71 ± 6.277 days with a range from 1 to 27 days, mean gestational age was 36.7±3.9 weeks, more than half of the neonates [94 (55.6%)] were term baby and rest [75 (44.4%)] were preterm.Mean weight was 2530.7±772.2 gm; among the enrolled neonates about two-third [101 (59.8%)] were of ≥2500gm, low birth weight (<2500-1500gm) were 41 (24.2%) and very low birth weight (<1500gm) were 27 (16%). In this study, more than half of the neonates [89 (53%)] were males and rest [80 (47%)] were female. Male: Female ratio was 1.1:1. (table I).
Characteristics (n=169) |
Mean ± SD |
Range |
Age (days) |
7.7±6.277 |
1-27 |
Length (cm) |
46.7±3.9 |
37-51 |
OFC (cm) |
32.9±2.7 |
25-36 |
Temperature (°F) |
98.5 ± 0.7 |
98-102 |
Heart rate (per min) |
133.1 ± 17.1 |
100-190 |
Respiratory rate (per min) |
53.4 ± 12.4 |
34-78 |
Among the enrolled neonates, most noted condition was chest indrawing, present in 70 (41.4%) neonates; followed by, pallor 46 (27.2%), nasal flaring 38 (22.5%), edema 32 (18.9%), Jaundice 34 (20.1%), grunting 30 (17.8%) and cyanosis 13(7.7%). Among the participants, 103 (60.9%) were getting oxygen, 34 (20.1%) were on ionotrops, 32(18.3%) were on phototherapy and 4 (2.4%) were on CPAP at the time of SpO2 measurements.
Each included patient had three paired SpO2 measurements from the wrist and ipsilateral palm and from the ankle and ipsilateral sole at 0sec, 30sec and at 1 min. The mean of three paired measurements of right palm and right wrist96.98± 1.52, 96.91 ± 1.65, left palm and left wrist 96.98 ±1.59, 96.93 ± 1.61, right sole and right ankle 97.40 ± 1.46, 97.51 ± 1.40, left sole and left ankle 97.58 ± 1.55, 97.02 ± 1.58 respectively (table II).
The linear relationship between SpO2 measured at right palm and right wrist was good (Correlation co-efficient r = 0.92; P<0.0001) and linear relationship of left palm with left wrist SpO2 measurement was also good (Correlation co-efficient r= 0.88; p <0.0001) (figure 1a, 1b).
Site of SpO2 Measurements |
Mean ± SD |
Range |
Right palm |
96.98± 1.52 |
92.66-99.33 |
Right wrist |
96.91 ± 1.65 |
92.00-99.33 |
Left palm |
96.98 ± 1.59 |
90.66-99.66 |
Left wrist |
96.93 ± 1.61 |
90.00-99.33 |
Right sole |
97.40 ± 1.46 |
92.00-99.33 |
Right ankle |
97.51 ± 1.40 |
92.33-99.00 |
Left sole |
97.58 ± 1.55 |
92.66-99.66 |
Left ankle |
97.02 ± 1.58 |
91.66-99.33 |
Similarly, the linear relationship between SpO2 measured at right sole and right ankle and SpO2 measured at left sole and left ankle showed good correlation (correlation co-efficientr= 0.90; p<0.0001; r= 0.98; p<0.0001) respectively (figure 1c, 1 d).
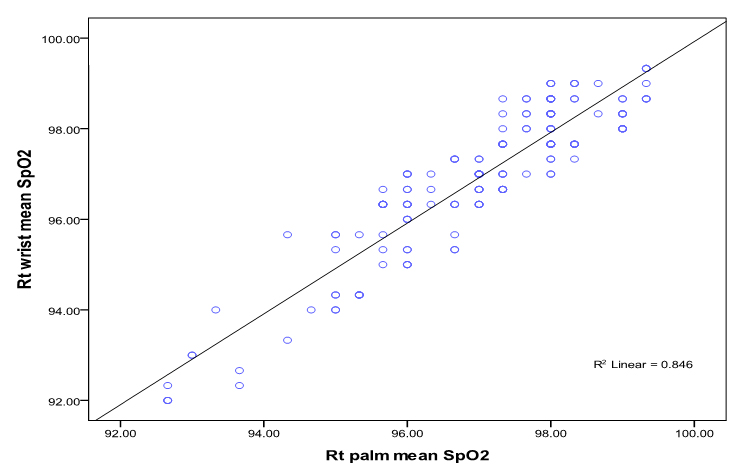
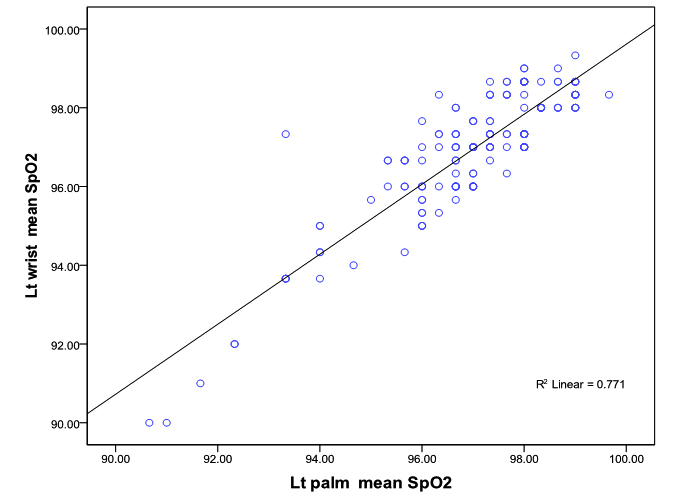
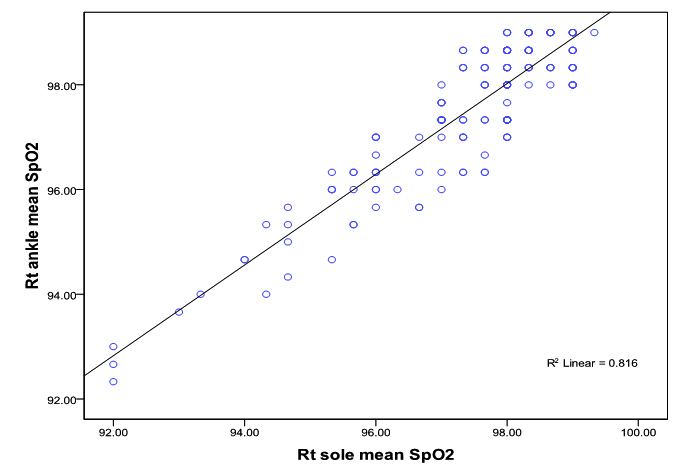
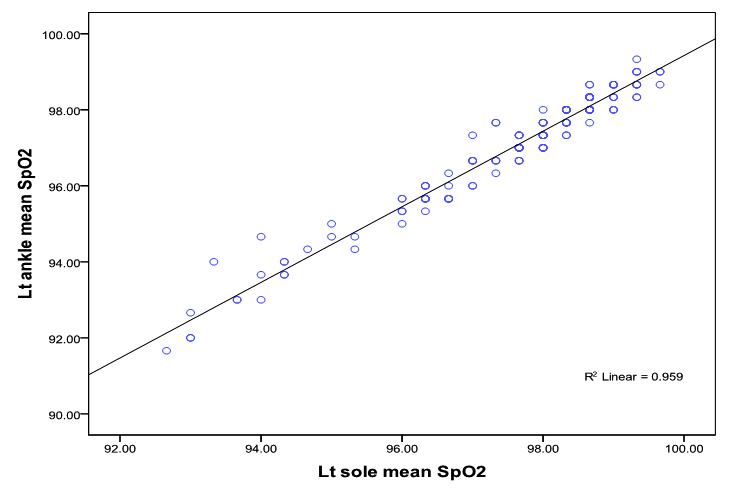
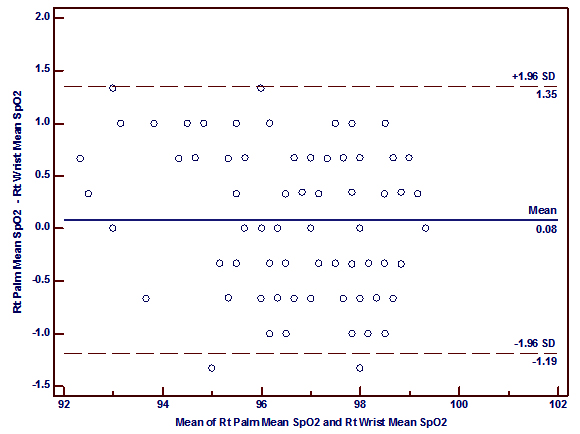
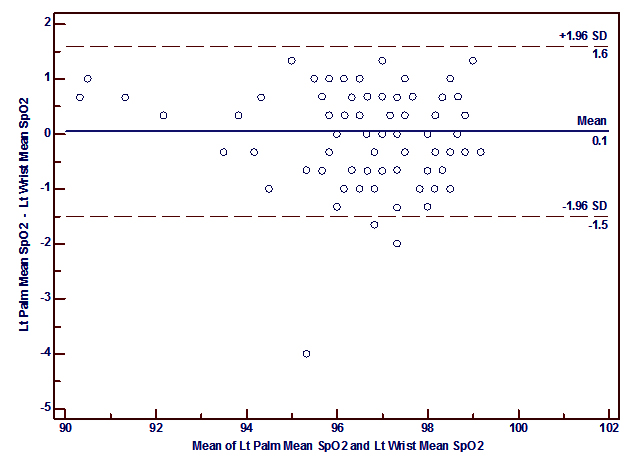
Bland-Altman plots of the mean differences (bias) and standard deviation of differences (precision) for the paired SpO2 measurements at the palms and the wrists showed a good agreement. The mean difference (bias) and the SD of the differences (precision) for SpO2 measurements at right palm and right wrist was 0.08 ± 0.65 and for the left palm and left wrist 0.05 ± 0.79% (figure 2 a, 2b).
Pulse oximetry is used on the basis of absorption of red and infrared light by oxygenated and deoxygenated hemoglobin. Two light emitting diodes send red and infrared light. Most modern commercial pulse oximeters work in the transmission mode, in which the selected site placed between emitting and receiving light diodes. A red and infrared light passes from one side of the sensor to the other. On the basis of the ratio of absorption of the red and infrared light caused by the difference in color between oxygen-bound (red) and unbound hemoglobin (blue) in the capillary bed, an estimation of oxygenation can be made. This requires the sensor to be applied on a relatively thin part of the anatomy, a fingertip or earlobe or, as in the case of an infant, the foot.12-14
One hundred sixty nine newborns admitted into the SCABU of the Dhaka Shishu Hospital during December 20012 to March 2013, had their pulse oximeter oxygen saturation (SpO2) measurement from probe placement at ipsilateral palm and wrist on both sides and ipsilateral sole and ankle on both sides. The accuracy of pulse oximeters is acceptable for a wide range of clinical applications. Most manufacturers claim that their instruments are accurate to within ±2% in the SpO2 range between 70 and 100% and ±3% for oxygen saturations between 50 and 69% and varies with the model of pulse oximetry.5,12
Interpretations of pulse oximetry readings account for a variety of factors that may artifactualy influence the results. Common sources of artifact include inappropriate probe placement, motion artifact, ambient light and electromagnetic radiation. In our study among 169 neonates, 32 (18.9%) were getting phototherapy. Chandler BD et alsuggest that phototherapy in vivo neither affects fetal erythrocytic affinity for oxygen nor causes haemolysis.15
Best location for pulse oximeter probe placement is a site with the best pulsatile vascular bed; the finger, toe, ear lobe, and bridge of the nose have been used. In neonates, flexible probes work through the palm, foot, penis, or arm. The cheek or wings of the nostril have also been used. However, overall performance of finger probes is generally found to be better than performance of probes at other sites.16Manufacturers generally recommend use of the foot for infants.17-19 Lyer et al reported no difference between hand and foot probe sites in infants less than 3 months of age undergoing cardiac surgery with cardiopulmonary bypass.12 Das J et al found that sole is the most accurate site of sensor location in cyanotic heart disease paediatric patients.20
Neonatal intensive care units have been using the ankle and the wrist as an alternative site to measure SpO2 in sick neonates. Although normal SpO2 values for neonates are available from the palms and sole, there are only one published data available for the wrist and ankle.10
It was to be found that the mean±sd SpO2 recorded from right palm, right wrist, left palm, left wrist, right sole, right ankle, left sole and left ankle were 96.98±1.52, 96.91±1.65, 96.98±1.59, 96.93±1.61, 97.40±1.46, 97.51±1.40, 97.58±1.55 and 97.02±1.58 respectively which were near similar to Phattraprayoon’s findings.10
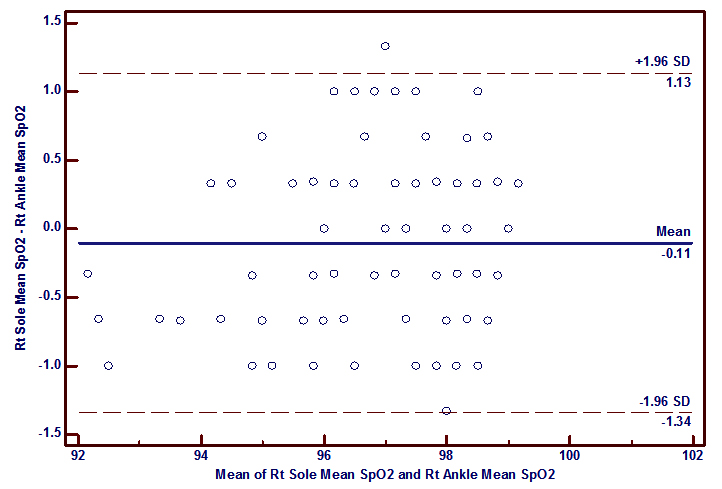
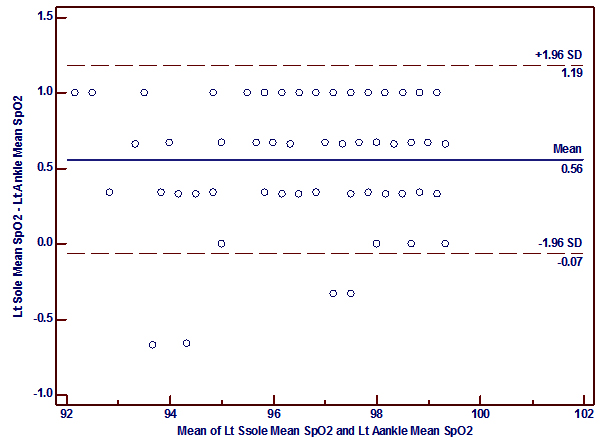
It was also to be found that a good correlation between SpO2 measured at the palm versus the wrist (r= 0.92, p<0.0001 (right); r= 0.88, p< 0.0001 (left)) and the sole versus the ankle (r=0.90, p<0.0001 (right); r= 0.98, p<0.0001 (left)) which is similar to the findings of Phattraprayoon N et al The Bland-Altman analysis shows that there was a good agreement of paired SpO2 measurements from these sites like that of Phattraprayoon’s findings.10 The bias and precision for SpO2 at the right palm and right wrist was 0.08 ± 0.65% and for the left palm and left wrist 0.05 ± 0.79%. Similarly, the bias and precision for SpO2 at the right sole and right ankle was -0.11 ± 0.63% and for the left sole and left ankle was 0.56 ± 0.32%.
Bilan N etalcompared the readings from3 different sites of pulse oximetry probe placements (ear, thumb andbig toe) and assessed their validity indetecting hypoxemia by comparing the results with ABG analysis. But in this study it couldn’t compare the measurements with ABG as it is costly.21
It was may be mentioned the study had some limitations that it was not compared the response time to get a reading from the pulse oximeter probe placed at different sites; the data were collected from stable patients only and there was no validation of SpO2 measured by pulse oximetry with arterial blood gas analysis.
Conclusion
This study revealed that there is good correlation and good agreement in paired SpO2 measurements from palm and wrist, sole and ankle. So, wrist and ankle can be used as alternative to routinely used palm and sole for SpO2 measurement.
Conflict of Interest
None
References
- Mower WR, Sachs C, Nicklin EL, Baraff LJ. Pulse oximetry as a fifth pediatric vital sign. Pediatrics 1997; 99: 681-86.
- Katzman GH. The newborn's SpO2: A routine vital sign whose time has come? Pediatrics 1995; 95:161-62.
- Sinex JE. Pulse oximetry: Principles and limitations. Am J Emerg Med. 1999; 17: 59-66.
- Hay WW, Brockway JM, Eyzaguirre M. Neonatal pulse oximetry: Accuracy and reliability. Pediatrics 1989; 83: 717-22.
- Durand M, Ramanathan R. Pulse oximetry for continuous oxygen monitoring in sick newborn infants. J Pediatr. 1986; 109: 1052-56.
- Fouzas S, Priftis KN, Anthracopoulos MB. Pulse oximetry in pediatric practice. Pediatrics. 2011;128: 740-52.
- Mengelkoch LJ, Martin D, Lawler J. A review of the principles of pulse oximetry and accuracy of pulse oximeter estimates during exercise. Phys Ther 1994; 74: 40-49.
- Ortega R, Hanson CJ, Elterman K, Woo A. Pulseoximetry. N Eng J Med 2011: 364; 33-36.
- Grap MJ. Pulse Oximetry.Crit Care Nurse 2002; 22: 69-76.
- Phattraprayoon N, Sardesai S, Durand M, Ramanathan R. Accuracy of pulse oximeter readings from probe placement on newborn wrist and ankle. Journal of Perinatology 2011; 32: 276-80.
- Villanueva R, Bell C, Kain ZN, Colingo KA. Effects of peripheral perfusion on accuracy of pulse oximetry in children. Journal of Clinical Anesthesia 1999; 11: 317-22.
- Iyer P, McDougall P, Loughnan P, Mee RB, Al-Tawil K, Carlin J. Accuracy of pulse oximetry in hypothermic neonates and infants undergoing cardiac surgery. Crit Care Med. 1996; 24: 507-11.
- World health organization. Pulseoximetry training manual:2011
- Kattwinkel J, Perlman JM, Aziz K, Colby C, Fairchild K, Gallagher J, Hazinski MF, Halamek LP, Kumar P, Little G, McGowan JE, Nightengale B, Ramirez MM, Ringer S, Simon WM, Weiner GM, Wyckoff M, Zaichkin J: Part 15: neonatal resuscitation: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010; 122 (18 suppl 3):S909-S919.
- Chandler BD, Cashore WJ, Monin PJ, Oh W. The lack of effects of phototherapy on neonatal oxygen dissociation curves and hemoglobin concentration in vivo. Pediatrics 1977; 59: 1027-31.
- Tittle M, Flynn MB. Correlation of pulse oximetry and co-oximetry. Dimens Crit Care Nurs.1997
- Kagle DM, Alexander CM, Berko RS, Giuffre M, Gross JB. Evaluation of the Ohmeda 3700 pulse oximeter: steady-state and transient response characteristics. Anesthesiology1987; 66: 376-80. 16: 88-95.
- Louis D, Sundaram V, Kumar P. Pulse oximeter sensor application during neonatal resuscitation: A randomized controlled trial. Pediatric 2014;133: 476-82
- LemaGE. Oral pulse oximetry in small children. Anesth Analg 1991; 72: 414.
- Das J, Aggarwal A, Aggarwal N K. Pulse oximeter accuracy and precision at five different sensor locations in infants and children with cyanotic heart disease. Indian journal of anesthesia 2010; 54:531-34.
- Bilan N, Behbahan A G, Abdinia B, Mahallei M. Validity of pulse oximetry in detection of hypoxaemia in children: Comparison of ear, thumb and toe probe placements. Eastern Mediterranean Health Journal 2010; 16: 218-22.